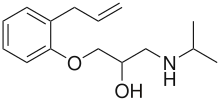
Alprenolol
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral |
| ATC code | |
| Pharmacokinetic data | |
| Protein binding | 80% - 90% |
| Elimination half-life | 2-3 hours → 4-OH-alprenolol |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank |
|
| ChemSpider |
|
| UNII | |
| KEGG |
|
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.033.750 |
| Chemical and physical data | |
| Formula | C15H23NO2 |
| Molar mass | 249.354 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
Alprenolol, or alfeprol, alpheprol, and alprenololum (Gubernal, Regletin, Yobir, Apllobal, Aptine, Aptol Duriles), is a non-selective beta blocker as well as a 5-HT1A and 5-HT1B receptor antagonist,[1] used in the treatment of angina pectoris.[2] It is no longer marketed by AstraZeneca, but may still be available from other pharmaceutical companies or generically.
References
- ↑ Langlois M, Brémont B, Rousselle D, Gaudy F (1993). "Structural analysis by the comparative molecular field analysis method of the affinity of beta-adrenoreceptor blocking agents for 5-HT1A and 5-HT1B receptors". Eur. J. Pharmacol. 244 (1): 77–87. doi:10.1016/0922-4106(93)90061-d. PMID 8093601.
- ↑ Hickie JB (1970). "Alprenolol ("aptin") in angina pectoris. A double-blind multicentre trial". Med. J. Aust. 2 (6): 268–72. doi:10.5694/j.1326-5377.1970.tb49984.x. PMID 4393977. S2CID 6879318.
| β, non-selective | |
|---|---|
| β1-selective | |
| β2-selective | |
| α1- + β-selective | |
| |
Serotonin receptor modulators | |||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-HT1 |
| ||||||||||||||||||||||||||||||||||||||
| 5-HT2 |
| ||||||||||||||||||||||||||||||||||||||
| 5-HT3–7 |
| ||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||
This article is issued from Wmcloud. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.