Butaperazine
 | |
| Clinical data | |
|---|---|
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number |
|
| PubChem CID | |
| DrugBank | |
| ChemSpider |
|
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.010.450 |
| Chemical and physical data | |
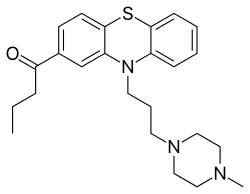
| Formula | C24H31N3OS |
| Molar mass | 409.59 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
Butaperazine (Repoise, Tyrylen) is a typical antipsychotic of the phenothiazine class.[2] It was approved in 1967, and possibly discontinued in the 1980s.
Synthesis
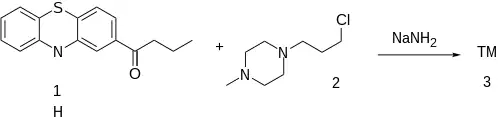
Patent:[3]
2-Butyrylphenothiazine [25244-91-1] (1) is the requisite starting material for carrying out the procedure. It is prepared in a manner that is synonymous with the method used in the propiomazine and propiopromazine already discussed. The 1-(γ-chloropropyl)-4-methylpiperazine [104-16-5] (2) is prepared in the conventional way from alkylating 1-methylpiperazine and 1-Bromo-3-chloropropane. Sodamide is used to extract the 10-H thereby facilitating the nucleophilic substitution reaction. And completing the instalment of the sidechain.
See also
References
- ↑ Anvisa (2023-03-31). "RDC Nº 784 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 784 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 2023-04-04). Archived from the original on 2023-08-03. Retrieved 2023-08-16.
- ↑ "Evaluation of a new antipsychotic agent. Butaperazine maleate (repoise maleate)". JAMA. 206 (10): 2307–8. December 1968. doi:10.1001/jama.206.10.2307. PMID 4386884.
- ↑ Dr Ulrich Hoerlein, Dr Klaus-Heinz Risse, Dr Wolfgang Wirth, DE 1120451 (1961 to Bayer Ag).
| Typical |
|
|---|---|
| Disputed | |
| Atypical |
|
| Others |
|
| |
Acetylcholine receptor modulators | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||
| D1-like |
| ||||||
|---|---|---|---|---|---|---|---|
| D2-like |
| ||||||
| |||||||
Histamine receptor modulators | |||||
|---|---|---|---|---|---|
| H1 |
| ||||
| H2 |
| ||||
| H3 |
| ||||
| H4 |
| ||||
| |||||
| Classes |
|
|---|---|
| Antidepressants (Tricyclic antidepressants (TCAs)) |
|
| Antihistamines |
|
| Antipsychotics |
|
| Anticonvulsants | |
| Anticholinergics | |
| Others |
|
This article is issued from Wmcloud. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.